|
Metric Measurement Notes Note: Prefixes in the metric system, from largest to smallest, are Mneumonic: King Henry Died By Drinking Chocolate Milk
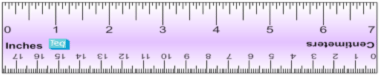
** 1 centimeter (cm) = 10 millimeters (mm) 1 millimeter (mm) = 1,000 micrometers (um) 1 meter (m) = 100 centimeters (cm)
To determine which way to move the decimal, follow the rule above. Move the decimal to the right (multiply) if converting from bigger units to smaller units. Move the decimal to the left (divide) if converting from small to bigger units. Metric Ruler
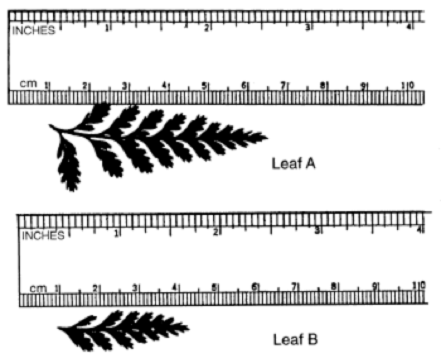
Note: Many times the end of a ruler will be worn away by student/teacher use or is inaccurate due to the manufacturing process. It is important to remember to take away the whole number increment. In this case, 1 cm from the measurement obtained.
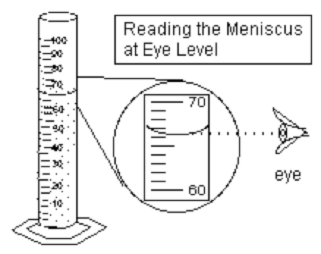
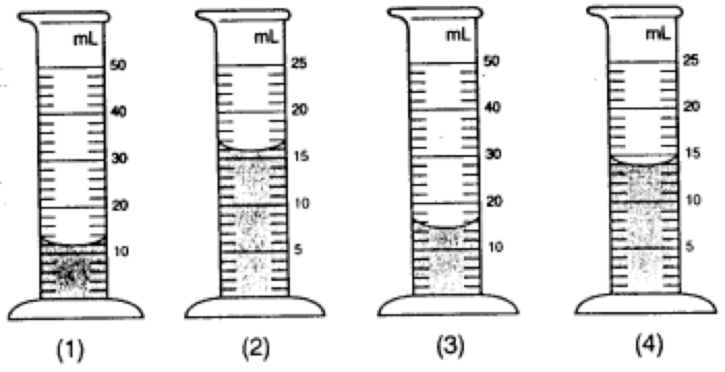
Answers: Leaf A in centimeters is 5.5 cm. In millimeters is 55 mm in length. Leaf B in centimeters is 3.3 cm. In meters is 0.33 m in length. The difference in length between leaf A and B in centimeters is 2.2 cm. Graduated Cylinder Note: When measuring liquids its usually in milliliters (ml). Remember to read at the bottom of the curved line or meniscus when measuring solutions involving water or most liquids. Read the meniscus at eye level! Usually, the surface of water in a glass graduated cylinder is curved. A plastic graduated cylinder has a flat meniscus.
Answers:
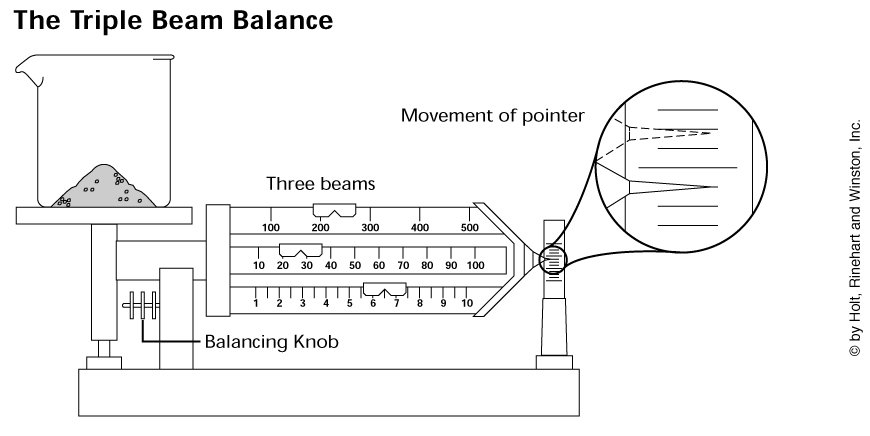
Triple Beam Balance Note: The triple beam balance measures the mass of an object. The unit of measurement is a gram. The balance has 3 beams (100 gram beam, 500 gram beam with units of 100 grams, and 10 gram beam with .10 gram units). To zero out the balance, all the weights need to be shifted to the left and the pointer should be straight. Make sure the pan/platform is empty and dried. Place object to measure on the pan/platform and move the 100 gram weight to the right first. Next, move the 10 gram weight if needed. Then the 1 gram weight if needed.
Answers:
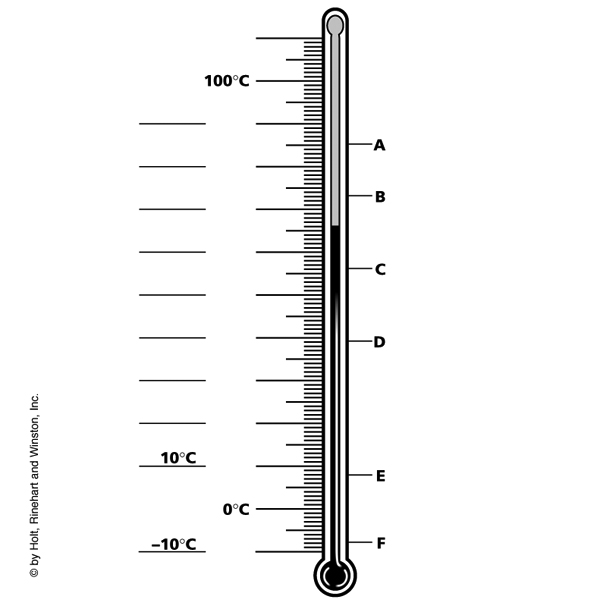
Thermometer Reading Note: Thermometers used to obtain the reading of heated liquids are long, thin glass cylinders with a bulb made of alcohol or mercury. When heated the liquid expands and the reading is measured in degrees Celsius. When measuring, the thermometer should be placed in the liquid alongside the beaker. A clip is used to suspend the bulb in the liquid. The bulb should Never rest on the bottom of the heated glass. Never hold the thermometer while heating the liquid. Read the final temperature once you have observed that the mercury has stopped rising. The mercury level that you read should be above the level of the liquid being heated.
Answers:
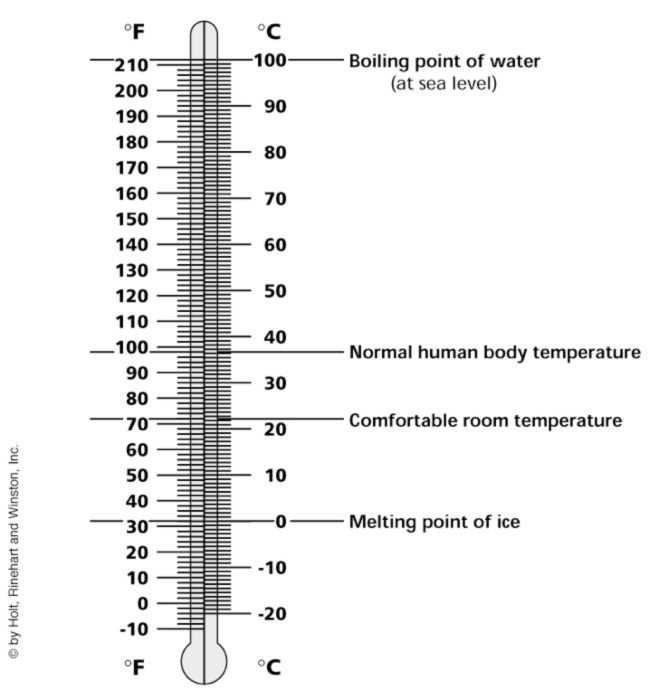
Celsius and Fahrenheit Scale Note: The freezing point of water is 0 degrees Celsius, the boiling point of water is 100 degrees Celsius, and normal human body temperature is 37 degrees Celsius.
Answers:
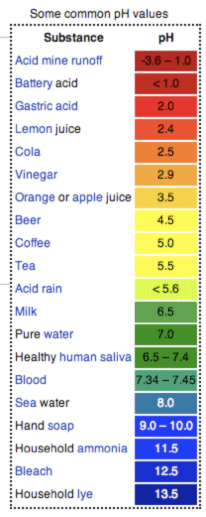
pH Measurement Note: A pH scale is a method used to determine if the chemical property of a solution is an acid or a base/alkaline. The indicator detects the hydrogen ions (H+) in a solution. An acidic solution has a high hydrogen ion (H+) concentration. A basic solution has a high hydroxide ion (OH-) concentration. The pH scale ranges from 0 to 14. A neutral solution has a pH value of 7. Water is a neutral solution because the water molecules exist in equilibrium with both H+ and OH- ions. An acidic solution has a pH value less than 7. A basic solution has a pH value greater than 7. For acids, each lower whole pH value is ten times stronger. For example, a pH of 2 is 10x stronger than a pH of 3 and 100x stronger than a ph of 4. For bases, each higher whole value is stronger. For example, a pH of 12 is 10x stronger than a pH of 11 and 100x stronger than a pH of 10. Review the pH Values of Common Items
Answers:
|