|
Chromatography Notes
Note: Chromatography is a technique used in a laboratory to separate the chemicals that make-up a liquid mixture. This method of separating colors can be analyzed when the end results show different colors on the paper.
This technique is used in police crime investigations to identify substances found in blood, urine, or drugs. It has even been utilized by food industries and in the study of plants to help identify compounds in those substances.
How Does Chromatography Work?
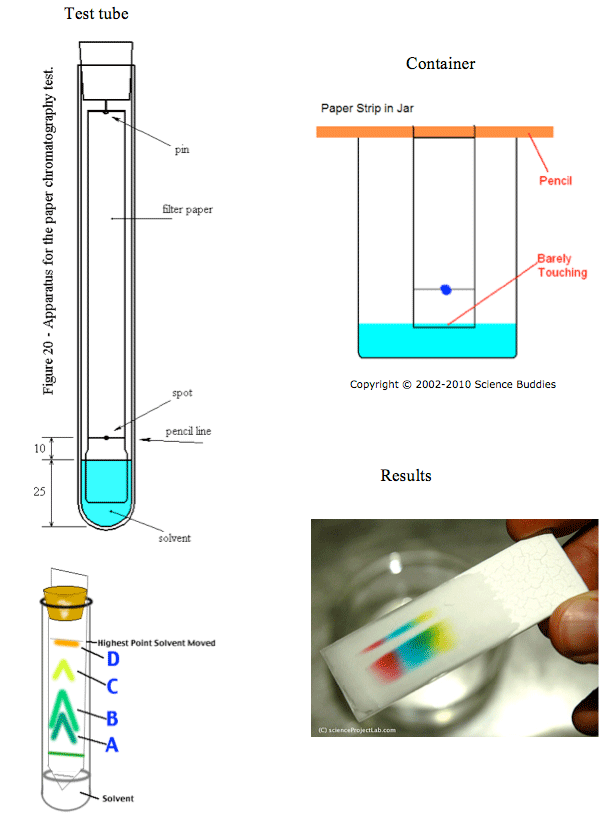
- A container is filled with a small amount of water called the solvent. This is also referred to as the mobile phase.
- The filter paper is called the stationary phase. This paper should be suspended in the middle of the container and not touching the sides of the container.
- The solvent level (water amount) should be below the line with the spot.
Therefore, only the tip of the filter paper is immersed in the solvent.
- The spot is a sample of the mixture that is being tested. The spot is the solute.
- Typically, the container or test tube is covered to prevent the solvent from evaporating as it travels up the paper. A closed container keeps the air saturated keeping the moisture of the water vapors from escaping.
- The solvent travels up the filter paper by a process called capillary action. This happens because the attraction of the water (adhesion) to the paper is stronger than the attraction of the water molecules to each other (cohesion). The resulting effect is water moving up the paper.
- When the solvent meets the solute, the mixture of solutes dissolves into the solvent creating a solution.
- Each solute will have a different attraction to the paper, to itself, and to the water molecules so the rate at which each solute travels will be different.
- The smaller molecules are faster and will travel a greater distance than larger molecules.
- The larger molecules are slower and will travel a shorter distance, staying lower on the filter paper.
- After about 15 minutes, analyze the different colors that separated from the original mixture. Which color travelled the furthest? The least furthest? How far did the solvent travel? Measure these distances with a ruler in cm.
Chromatography Illustrations
Adapted from http://biobuncic.org/Chromatograhy.htm
|
